Brian J. Hales

Position: Professor Emeritus – Bioinorganic, Spectroscopy
BS: Chemistry; Carnegie Institute of Technology, 1966
PhD: Physical Chemistry; with James R. Bolton; University of Minnesota, 1970
PostDoc: with Sir George Porter; Royal Institution of Great Britain, 1970-71; NIH Postdoctoral Fellow; with Paul Loach; Northwestern University, 1971-73
Phone: (225) 578-4694
Fax: (225) 578-3458
E-mail: bhales@lsu.edu
Office: 410 Choppin Hall
Areas of Interest
Trace metals have been known for a long time to play many important roles in biology. Our research investigates one of those roles, i.e., the function of redox active metals and metal clusters in metalloproteins. Metalloproteins constitute some of the most important class of enzymes. Many of these enzymes are involved with the fundamental reactions needed for life on this planet, e.g., respiration, photosynthesis and nitrogen fixation. In these reactions metals or metal clusters are often at the active site.
Currently our group is investigating one of those processes, nitrogen fixation, which catalyzes the fixation or reduction of N2 into ammonia:
N2 + 8H+ + 8e- > NH3 + H2
Over half of the fixed nitrogen on earth comes from nitrogen fixation while the remainder is produces by either chemical fertilizers (i.e., Haber-Bosch process) or generated in the atmosphere by lightening.
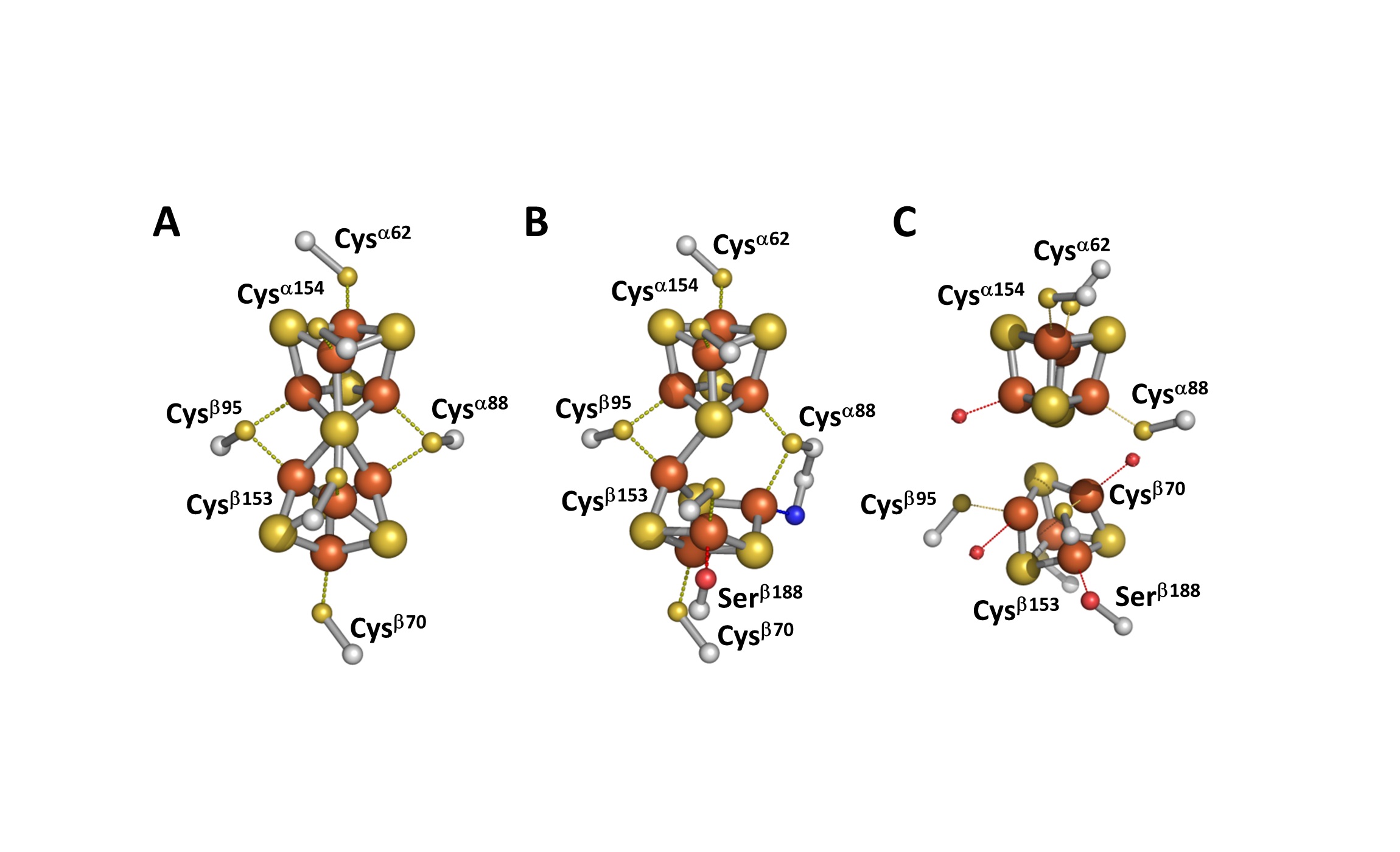
The nitrogen fixing enzyme, Nitrogenase, contains two of the most complex metal clusters found in Nature, the P-cluster (Fe8S7), which has an electron transfer function, and the FeMo cofactor (MoFe7S9C-Homocitrate), which serves as the enzyme’s active site (Figure 1).
Using a combination of Magnetic Circular Dichroism (MCD) spectroscopy and Electron Paramagnetic Resonance (EPR) spectroscopy, we are investigating the biosynthetic pathway of both nitrogenase clusters. MCD spectroscopy records the magnetic-field induced CD spectrum. While all substances possess an MCD spectrum, only paramagnetic substances yield an intense spectrum. In the case of paramagnetic FeS proteins, the MCD spectrum can be directly related to the FeS cluster type (e.g., [Fe2S2], [Fe4S4]) and spin state.
EPR spectroscopy is always used in conjunction with MCD spectroscopy. Both spectra are complimentary in that they rely on the same parameters (i.e., the spectroscopic g factor and spin state).
The results of these studies will be of great interest to inorganic chemists as well as to the section of the chemical industry focused on the synthesis of inorganic catalysis.
Figure 1: Structure of the P-cluster in the (A) reduced (PN) and (B) oxidized (P2+) states. Also shown is the proposed structure of [Fe4S4]-like clusters in the P-cluster precursor protein, DnifH NifDK (C).
As an example of the breath of information that can be obtained the synthesis of the nitrogenase P-cluster was investigated. The biosynthesis of the P-cluster starts with the precursor protein, DnifH NifDK. The DnifH NifDK protein contains neighboring pairs of [Fe4S4]-like clusters positioned at the site of the eventual P-cluster. Our lab uses two techniques, Electron Paramagnetic Resonance (EPR) and Magnetic Circular Dichroism (MCD) spectroscopy, to study the P-cluster biosynthesis. EPR spectroscopy is analogous to NMR spectroscopy except it monitors the electron spin in a paramagnetic material rather than the nuclear spin of certain isotopes. MCD spectroscopy records the CD spectrum of a sample induced by a magnetic field. Both spectroscopic techniques are especially helpful in monitoring the electronic structure of FeS clusters.
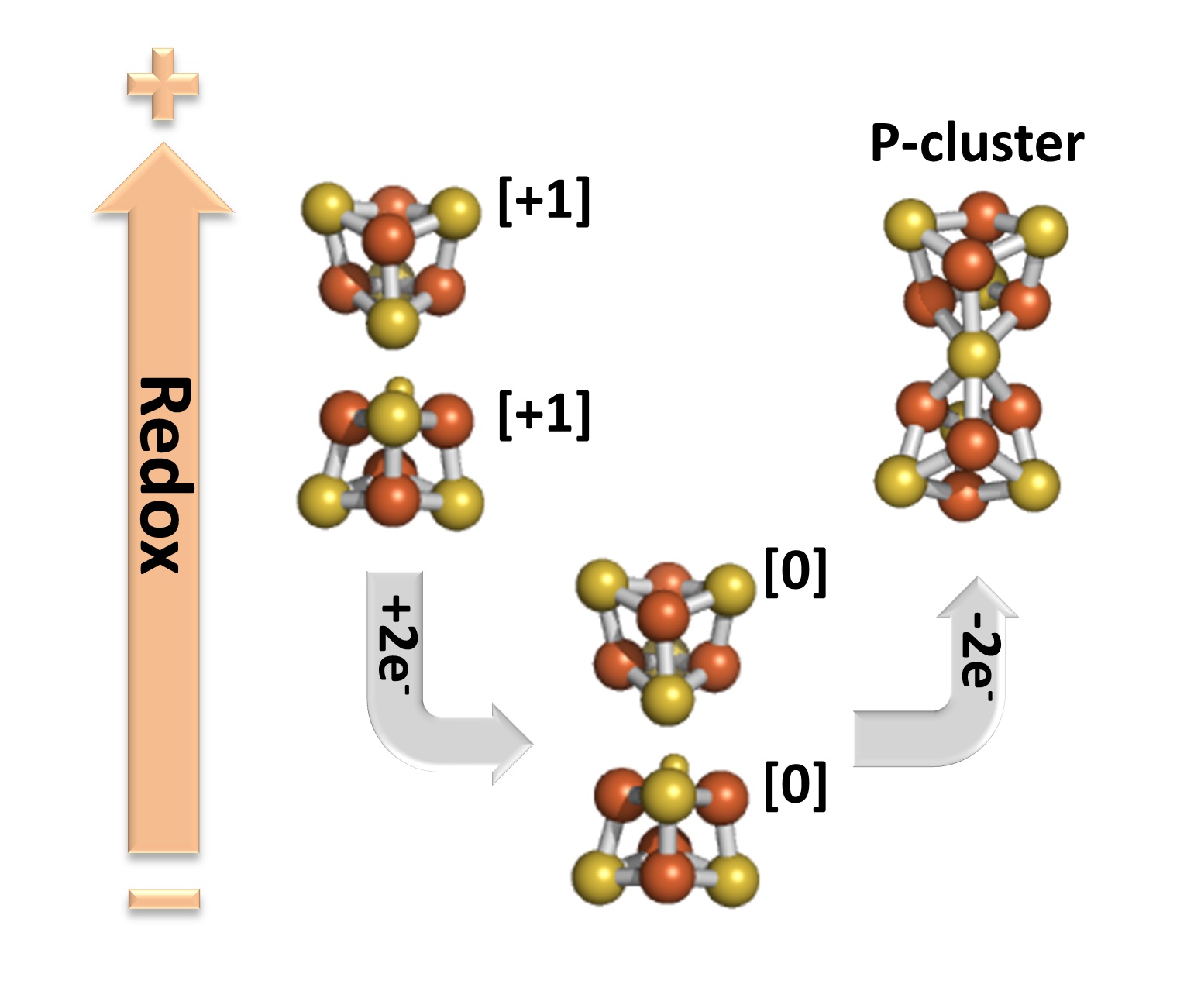
EPR and MCD spectroscopic studies revealed several exciting and surprising properties of the clusters in the DnifH NifDK protein. First, the clusters are the first [Fe4S4] clusters to be paramagnetic in the oxidized (2+) state. Second, the clusters are only the third [Fe4S4] protein clusters that can exist in the all-ferrous (0) reduced state. Most surprising, chemical oxidation of the all-ferrous cluster resulted in the formation of P-clusters in a non-enzymatic reaction (Figure 2).
Figure 2: Redox cycle showing the path of the non-enzymatic synthesis of the P-cluster from the two [Fe4S4] clusters in the DnifH NifDK protein.
Honors and Awards
Les & Dot Broussard Alumni Association Departmental Professor (2006)
LSU Distinguished Research Master Award (2004)
Chairman of Gordon Conference on Nitrogen Fixation (July, 2002)
Elected AAAS Fellow (2001)
Council Member for the Gordon Research Conferences (2001-2002)
LSU Alumni Faculty Excellence Award (1996)
Elected to Sigma Xi (1979)
Phi Kappa Phi Award of Merit for Research (1978)
Recent Publications
-
An EPR and VTVH MCD spectroscopic investigation of the nitrogenase assembly protein NifB” Kresimir Rupnik, Lee Rettberg, Kazuki Tanifuji, Johansse G. Rebelein, Markus M. Ribbe, Yilin Hu and Brian J. Hales J. Biol. Inorg. Chem. 2021, 26, 403-412.
-
Electron Paramagnetic Resonance and Magnetic Circular Dichroism Spectra of the Nitrogenase M Cluster Precursor Suggest Sulfur Migration upon Oxidation: A Proposal for Substrate and Inhibitor Binding” Kresimir Rupnik, Kazuki Tanifuji, Lee Rettberg, Markus M. Ribbe, Yilin Hu and Brian J. Hales ChemBioChem 2020, 21, 1767-1772.
-
Kresimir Rupnik, Chi Chung Lee, Yilin Hu, Markus W. Ribbe and Brian J. Hales, “A VTVH MCD and EPR Spectroscopic Study of ‘Maturation’ of the Second Nitrogenase P-Cluster”, Inorg. Chem., 2018,
-
Brian J. Hales “Ethylene Glycol Quenching of Nitrogenase Catalysis: An Electron Paramagnetic Resonance Spectroscopic Study of Nitrogenase Turnover States and CO Bonding”, Biochemistry 2015, 54, 4208-4215.
-
Kresimir Rupnik, Chi Chung Lee, Jared A. Wiig, Yilin Hu, Markus W. Ribbe, and Brian J. Hales, “Nonenzymatic Synthesis of the P-Cluster in the Nitrogenase MoFeProtein: Evidence of the Involvement of All-Ferrous [Fe4S4]0 Intermediates” Biochemistry, 2014, 53, 1108-1116.
-
Kresimir Rupnik, Yilin Hu, Chi Chung Lee, Jared A. Wiig, Markus W. Ribbe, and Brian J. Hales, “P+ State of Nitrogenase P-Cluster Exhibits Electronic Structure of a [Fe4S4]+ Cluster” J. Amer. Chem. Soc. 2012, 134, 13749-13754.
-
Rupnik, K., Lee, C. C., Hu, Y., Ribbe, M. W., and Hales, B. J. “[4Fe4S]2+ Clusters Exhibit Ground-State Paramagnetism” J. Amer. Chem. Soc. 2011, 133, 6871-6873.
-
Kresimir Rupnik, Yilin Hu, Aaron W. Fay, Markus W. Ribbe and Brian J. Hales, “Variable-Temperature, Variable-Field Magnetic Circular Dichroism Spectroscopic Study of NifEN-Bound Precursor and “FeMoco”” Biol. Inorg. Chem. 2011, 325-332
-
Marcia S. Cotton, Kresimir Rupnik, Robyn B. Broach, Yilin Hu, Aaron W. Fay, Markus W. Ribbe and Brian J. Hales, “VTVH-MCD Study of the ∆nifB∆nifZ MoFe Protein from Azotobacter vinelandii”, J. Amer. Chem. Soc. 2009, 131, 4558-4559.
-
Broach, R. B.; Rupnik, K.; Hu, Y.; Fay, A. W.; Cotton, M.; Ribbe, M. W.; Hales, B. J. “Variable-Temperature, Variable-Field Magnetic Circular Dichroism Spectroscopic Study of the Metal Clusters in the ∆nifB and ∆nifH MoFe Proteins of Nitrogenase from Azotobacter vinelandii”, Biochemistry 2006, 45, 15039-1504.
Former Ph.D. Students
Anupam Gupta PhD, Bell Labs
Karen Howard PhD, Procter & Gamble
Melinda Oliver PhD
Ronald Tittsworth PhD, LSU CAMD
Carol Blanchard PhD, Postdoc
Linda Cameron PhD, Postdoc